OPIOID ADDICTION
Listen to the audio version of this article:
Developed in the 1960’s and approved for medical use in 1981, Buprenorphine was approved in 2002 by the Food and Drug Administration (FDA) for treatment of opioid dependency. It attaches to the opioid receptors in the brain and alleviates withdrawal. People take this medication soon after stopping opioid use, even as soon as 24 hours after the last painkiller or heroin use. Because the med acts quickly, the patient often reaches a stable baseline in a few days.
Opioid Replacement 2.0
Prior to buprenorphine’s approval and regulation by the Drug Enforcement Administration (DEA), the primary medication used to treat heroin addiction was methadone. The methadone approach continues to have the same goals as that of today’s buprenorphine programs:
- Help alleviate opioid withdrawal symptoms
- Substitute heroin with a safer, longer-acting opioid version
- Provide monitoring and accountability
- Adjust dosing and address side effects
- Use therapy modalities to create life change
- Potentially taper the methadone
The biggest clinical difference between methadone and buprenorphine is that of a ‘ceiling’ effect. Buprenorphine has a maximum response which seems to be around 24 mg. Response above 24mg plateaus off, and most people are fully stabilized at a dose of 16mg per day. Risk of respiratory depression is almost non-existent, even with a buprenorphine overdose.
This is not the case with methadone, as methadone is a full opioid agonist. With continual dose increases of methadone, the response is linear and will ultimately lead to respiratory depression and death.
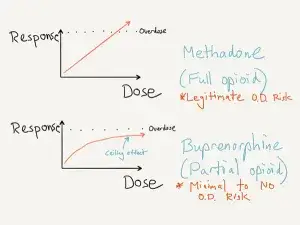
As a result, we consider methadone a more lethal medication.
Office-Based Treatment
Another important distinction between approaches is the office-based nature of the prescribing. Because of the ceiling effect discussed above, buprenorphine is safer in outpatient settings. The person receives a prescription for the medicine and fills it at a local pharmacy. This is not the case with methadone programs, as that dosing actually occurs on-site.
With this comes significant life issues and difference in patient populations. As methadone patients are dosing at a clinic daily, there is a positive benefit of close contact with nursing and medical staff. As a result of the contact with others, the person has potentially greater exposure to drug use from people in the clinic as well.
Keeping in line with methadone requirements, the DEA has a training program that all physicians must complete if they wish to prescribe buprenorphine. Through this training, the physician earns a second DEA number for the specific purpose of prescribing the buprenorphine. DEA agents my periodically visit those physicians to verify quality prescribing practices and adherence to guidelines.
A physician cannot legally prescribe methadone for the purpose of opioid addiction in an outpatient setting. The physician can prescribe methadone for chronic pain management.
Dose Ranges for Buprenorphine
Buprenorphine comes in a sublingual form and is available as both a tablet or film (Suboxone brand name). It is in dose increments of 2mg to 8mg. Standard dose ranges for buprenorphine are between 8mg and 16mg per day, with some patients feeling stable at doses less than 8mg and some patients requiring up to 24mg daily.
Although this medication is FDA approved up to a total daily dose of 32mg, this seems in far excess of what people need to maintain stability. A savvy clinician would raise questions of diversion risk or misuse of the medication for a person requesting or ‘requiring’ 32mg daily.
Blockade Component
The buprenorphine molecule binds very tightly to the opioid receptors in the brain. This is one of the primary reasons we use it for addiction treatment – it essentially blocks out opioid use. Because of the intensity of the binding, it is common for people to experience precipitated withdrawal symptoms when the buprenorphine displaces heroin from the receptor. This phenomenon is likely not harmful to the body but can be quite miserable. We work closely with people to help them bridge onto buprenorphine treatment in a safe and comfortable manner.
The brand name Suboxone is a combination med including buprenorphine and naloxone. This is in a 4:1 ratio of dosing. Naloxone is an opioid overdose reversal agent and causes immediate withdrawal when given to overdose victims. Contrary to popular belief, naloxone is not the source of the blockade effect with Suboxone. In fact, the naloxone is essentially inactive for people on maintenance Suboxone.
The rationale behind adding an inactive ingredient to the buprenorphine during the development of Suboxone was to prevent the intravenous use of the med. When patients administer the Suboxone in such a fashion, the naloxone will take effect, causing overwhelming withdrawal symptoms.
Craving Reduction
Buprenorphine reduces cravings to use heroin, decreases heroin use, increases treatment retention, and lowers the rate of death. As a result of strong clinical evidence, we describe buprenorphine as a Tier 1 medication.
Access and Referral Options for Treatment
The infrastructure for buprenorphine treatment is quite extensive. This med is now the standard of care for outpatient opioid addiction treatment and stabilization. When searching for a treatment provider, there a few options to consider. A person could seek out an advanced addiction treatment center, a private addiction specialist, or a primary care provider. The more dedicated to addiction treatment your provider, the more therapeutic value you would receive in addition to medications. If your provider only focuses on the buprenorphine, you may need to seek out counseling and comprehensive addiction support in addition to the prescription.
Read more CeDAR Education Articles about Opioid Addiction including Moving from Painkillers to Heroin.
